O-Toluidine is a synthetic light sensitive light yellow liquid that is slightly soluble in water and miscible with carbon tetrachloride diethyl ether and ethanolThe hydrochloride is a synthetic light sensitive white crystalline powder that is soluble in dimethylsulfoxide and ethanol. They will not be considered in the grading.

Draw The Structure S Of The Major Organic Product S You Would Expect From Reaction Of M Toluidine M Methylaniline Homeworklib
Do not include lone pairs in your answer.
. You do not have to consider stereochemistry. The purpose of this note is twofold. All three are aryl amines whose chemical structures are similar to aniline except that a methyl group is substituted onto the benzene ring.
There are three isomers of toluidine which are organic compounds. They will not be considered in the grading. 2 The chemical formula for o-toluidine is C H N and it has a molecular weight of 10715 gmol.
You do not have to explicitly draw H atoms. You do not have to consider stereochemistry. Decreasing order of basicity.
Structure of o-toluidine C 14 H 16 N 2. Do not include lone pairs in your answer. You do not have to consider stereochemistry.
Answer 1 of 3. Draw the structure s of the major organic product s you would expect from reaction of m-toluidine m-methylaniline with HNO and HjSO followed by KCN and CuCN. To draw attention to the wealth of structure revealed by toluidine blue O when it is used to stain fresh or fixed plant tissues and to outline very simple and rapid procedures for obtaining temporary or permanent mounts of stained sections.
See the answer See the answer done loading. You do not have to consider stereochemistry. Draw the structure s of the major organic product s you would expect from reaction of m-toluidine m-methylaniline with HCl 1 equivalent.
28 The vapor pressure for o-toluidine is 031 7 7 9. Vapor-phase m-anisidine will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals. It is a colorless liquid although commercial samples are often yellowish.
Therefore it will undergo direct photolysis by sunlight. P toluidine o toluidine m toluidine aniline Basicity depends on the ability of electron donating groups that increases basicity of the lone pair of nitrogen. O-Toluidine is a colorless to light yellow liquid.
Draw the structure s of the major organic product s you would expect from reaction of m -toluidine m -methylaniline with CH3I excess. Compound m-Toluidinewith free spectra. Do not include lone pairs in your answer.
7 NMR 7 FTIR 1 Raman 2 Near IR and 5 MS. These isomers are o-toluidine m-toluidine and p-toluidine with the prefixed letter abbreviating respectively ortho. We review their content and use your feedback to keep the quality high.
You do not have to explicitly draw. 2 o-Toluidine has an odor threshold of 025 parts per million ppm. The half-life for this reaction in air is estimated to be 19 hours.
Aniline is lesser basic tha. M-Anisidine absorbs light at 285 nm. It is a precursor to.
51 NMR 11 FTIR 1 Raman 2 Near IR and 10 MS. 7 o-Toluidine is slightly soluble in water. Compound N-ethyl-m-toluidinewith free spectra.
Draw the structure s of the major organic product s you would expect from reaction of m-toluidine m-methylaniline with HCl 1 equivalent. You do not have to explicitly draw H atoms. Download Hi-Res Image Download to MS-PowerPoint Cite This.
THE STRUCTURE OF THE SO-CALLED TOLUIDINE BLUE The Journal of Organic Chemistry. You do not have to explicitly draw H atoms. M-toluidine - cas 108-44-1 synthesis structure density melting point boiling point.
Donating effect is most powerful on para then on ortho. They will not be considered in the grading. It is the most important of the three isomeric toluidines.
This problem has been solved. M-Toluic acid C8H8O2 CID 7418 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Repeated determination Download PDF.
Experts are tested by Chegg as specialists in their subject area. Up to 10 cash back Structure of o-toluidine C 14 H 16 N 2. O-Toluidine and o-toluidine hydrochloride are used primarily as intermediates in the manufacture of dyes.
We investigate the structural features of the polyaniline derivative polyo-toluidinePOT. M-toluidine appears as a clear colorless liquid. Flash point below 200F.
Due to the activation of the ring by the amino group it is Ortho para directing and hence methyl group on the para position which is not a dominant electrophile Directs the electron towards the ring and and the ring becomes activated. O-Toluidine ortho-toluidine is an organic compound with the chemical formula CH 3 C 6 H 4 NH 2. In the base form POT is amorphous with an X-ray diffraction pattern.
Draw the structure s of the major organic product s you would expect from reaction of m-toluidine m-methylaniline with HCl 1 equivalent. Draw the structures of the major organic products you would expect from reaction of m-toluidine m-methylaniline with HCl 1 equivalent. N-Methyl-m-toluidine C8H11N CID 69675 - structure chemical names physical and chemical properties classification patents literature biological activities.
Who are the experts. 1941 06 5 732-749.

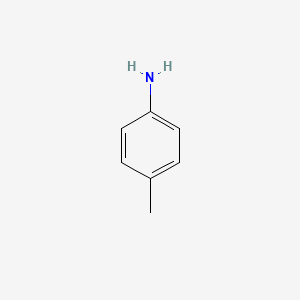
P Toluidine C6h4ch3nh2 Pubchem
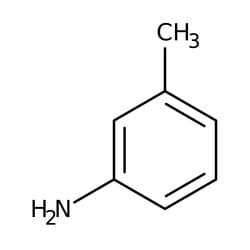
M Toluidine 99 Thermo Scientific

Give The Structures Of The Major Organic Products You Would Expect From Reaction Of M Toluidine M Methylaniline Homeworklib
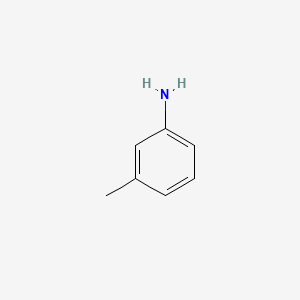
M Toluidine C6h4ch3nh2 Pubchem

Solved Draw The Structure Of M Toluidine Sn3 Hn Ch3 Chegg Com

M Toluidine Structure C7h9n Over 100 Million Chemical Compounds Mol Instincts
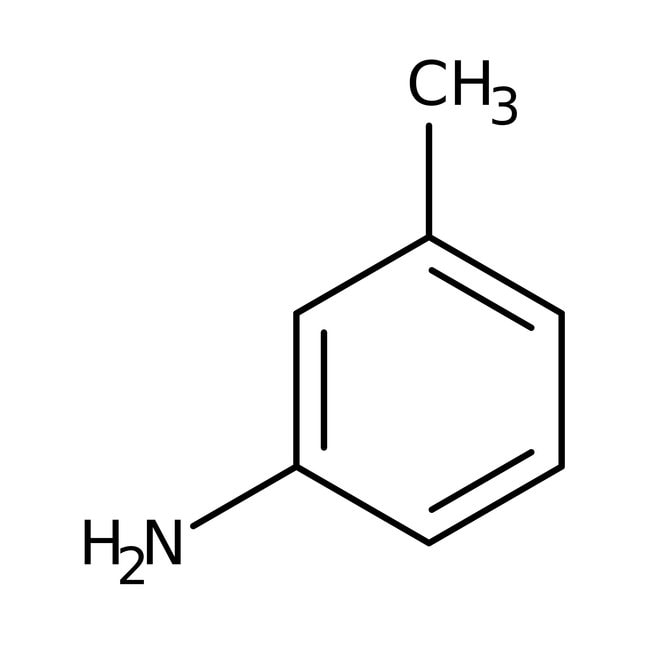
0 comments
Post a Comment